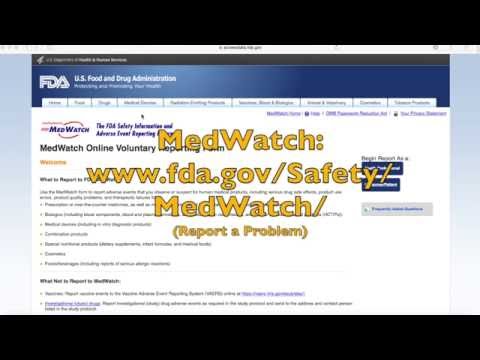
Today, I am going to show you just how easy it is to report an unhealthy reaction to a personal care product to the Food and Drug Administration (FDA). An unhealthy adverse reaction to your product can be anything from a simple rash or a headache to more serious impacts like hospitalization or permanent damage. Reporting these reactions to the FDA is so important because it gives them a heads up that a particular product might be causing health problems. The FDA really wants to know because they need official reports like this in order to take action or conduct further investigations. The information you provide can help flag problem products and chemicals and may be used during inspections of the manufacturers' facilities. While you may not see an immediate action from the FDA after filing your report, over time, your information combined with reports from others can really make a difference to the safety of products that we use every day. So, here's how it works - the FDA has an online reporting system called MedWatch. To find MedWatch, you can go to this URL or simply go to any search engine and type in "MedWatch online voluntary reporting system." It will be the first link that pops up, and it should direct you to a page with the heading "FDA US Food and Drug Administration." Next, you'll want to head to the right side of the screen to begin the report. You'll want to select the second option, which is "consumer or patient." Clicking on that will take you to the first of five short pages of questions. On the first page, you'll need to answer what kind of problem it was that you experienced. For example, if you had a problem with a feminine wash and got a rash from using...
Award-winning PDF software





Fda 3971 Form: What You Should Know
Download Federal Form for Application for Small Business (3971) (US) for instructions on how to file Form 3971. Form 3760: Application for Special Taxpayer Identification Number (SKIN) — FDA, OMB Docket No. 4. Download the State Department of Revenue “Form 3760” and the US Tax Court “Form 3760” for Instructions on how to apply for STN. Form 3863: Application for Nonpublic Financial Disclosure Certificate (NFC) — FDA, OMB Docket No. 4. Download State Department of Revenue “Form 3863” and US Tax Court “Form 3863” for Instructions on how to apply for NFL. Form 8300: Unexpired Individual Medical Certificate — FDA, OMB Docket No. 4. Download State Department of Revenue “Form 8300”, US Tax Court “Form 8300” and the IRS “Forms 8815 and 883” for Instructions on how to apply for Unused Medical Certificate. Form 9099-Q: Qualified Health Insurance Offer (HI). FDA, OMB Docket No. 4. Download the IRS “Form 9099: Qualified Health Insurance Offer (HI).” Form 8974: Application for Health Coverage — FDA, OMB Docket No. 4. Download the State Department of Revenue “Form 8974: Affiliate Application for Health Coverage (HCC). Form 8974EZ: Affiliate Application for Health Coverage (EZ) (Non-Exempt)” FDA, OMB Docket No. 4. Download the State Department of Revenue “Form 8974EZ: Affiliate Application for Health Coverage (EZ) (Non-Exempt)” for instructions on how to file the Form 8974EZ. Form 8974T: Affiliate Application for Health Coverage (T)” FDA, OMB Docket No. 4. Download the State Department of Revenue “Form 8974T: Affiliate Application for Health Coverage (T)” and US Tax Court “Form 8974T” for instructions on how to file the Form 8974T. Form 8962: Application for Certificate of Eligibility for Group Health Plan (PHP): Medicare — FDA, OMB Docket No. 4.
online solutions help you to manage your record administration along with raise the efficiency of the workflows. Stick to the fast guide to do Form 3911, steer clear of blunders along with furnish it in a timely manner:
How to complete any Form 3911 online: - On the site with all the document, click on Begin immediately along with complete for the editor.
- Use your indications to submit established track record areas.
- Add your own info and speak to data.
- Make sure that you enter correct details and numbers throughout suitable areas.
- Very carefully confirm the content of the form as well as grammar along with punctuational.
- Navigate to Support area when you have questions or perhaps handle our assistance team.
- Place an electronic digital unique in your Form 3911 by using Sign Device.
- After the form is fully gone, media Completed.
- Deliver the particular prepared document by way of electronic mail or facsimile, art print it out or perhaps reduce the gadget.
PDF editor permits you to help make changes to your Form 3911 from the internet connected gadget, personalize it based on your requirements, indicator this in electronic format and also disperse differently.
Video instructions and help with filling out and completing Fda form 3971